Redox-neutral photochemical Heck-type arylation of vinylphenols activated by visible light†
Abstract
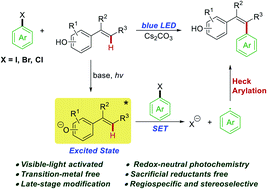
Disclosed herein is a photochemical Heck-type arylation of vinylphenols with non-activated aryl and heteroaryl halides under visible light irradiation. Preliminary mechanistic studies suggested that the colored vinylphenolate anions acted as a strong reducing photoactivator to directly activate (hetero)aryl halides without the need for any sacrificial reductants. The photochemically generated aryl radicals coupled with another molecule of vinylphenol to afford the Heck-type arylation product in a regiospecific and stereoselective manner. The developed photochemical arylation protocol showed exceptional functional group tolerance and was successfully applied in the challenging late-stage modification of natural products without any protection–deprotection procedures.


 Please wait while we load your content...
Please wait while we load your content...