Enantioselective oxygenation of exocyclic methylene groups by a manganese porphyrin catalyst with a chiral recognition site†
Abstract
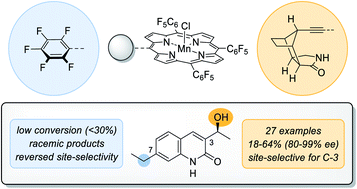
The natural enzyme cytochrome P450 is widely recognised for its unique ability to catalyse highly selective oxygen insertion reactions into unactivated C–H bonds under mild conditions. Its exceptional potential for organic synthesis served as an inspiration for the presented biomimetic hydroxylation approach. Via a remote hydrogen bonding motif a high enantioselectivity in the manganese-catalysed oxygenation of quinolone analogues (27 examples, 18–64% yield, 80–99% ee) was achieved. The site-selectivity was completely altered in favour of a less reactive but more accessible position.


 Please wait while we load your content...
Please wait while we load your content...