Hydrophosphination of boron–boron multiple bonds†
Abstract
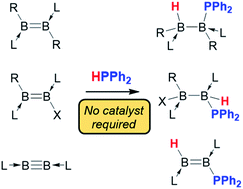
Five compounds containing boron–boron multiple bonds are shown to undergo hydrophosphination reactions with diphenylphosphine in the absence of a catalyst. With diborenes, the products obtained are highly dependent on the substitution pattern at the boron atoms, with both 1,1- and 1,2-hydrophosphinations observed. With a symmetrical diboryne, 1,2-hydrophosphination yields a hydro(phosphino)diborene. The different mechanistic pathways for the hydrophosphination of diborenes are rationalised with the aid of density functional theory calculations.


 Please wait while we load your content...
Please wait while we load your content...