Acyl azide generation and amide bond formation in continuous-flow for the synthesis of peptides†
Abstract
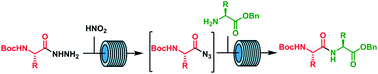
The development of new protocols for peptide bond formation which avoid side reactions, including epimerization, are very important. The acyl azide method for the preparation of peptides is very good at maintaining chiral integrity, however; acyl azides are considered to be highly unstable and potentially explosive intermediates. Thus, the acyl azide method is underutilized by the synthetic community. Acyl azides were safely generated and reacted in situ within a continuous-flow system. The acyl azide was generated by using nitrous acid in water, and efficiently extracted into the organic phase containing the amine nucleophile for peptide coupling. The protocol has been used to prepare a number of dipeptides (5 examples) without epimerization (<1%). A tripeptide, D-Ala-D-Ala-L-Ala-OBn, was also synthesized.


 Please wait while we load your content...
Please wait while we load your content...