Pyrolysis of mixtures of methane and ethane: activation of methane with the aid of radicals generated from ethane
Abstract
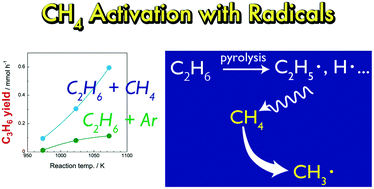
Direct chemical conversion of methane (CH4) has been actively researched in order to use natural gas as a chemical resource. However, the high stability of CH4 molecules hinders the chemical conversion of CH4. In this study, we investigated pyrolysis of mixtures of CH4 and ethane (C2H6) at 973–1073 K. Even though CH4 alone did not react in the temperature range, mixtures of CH4/C2H6 and of Ar/C2H6 showed different pyrolysis behaviours; the co-existence of CH4 significantly increased yields of propylene (C3H6), propane (C3H8) and toluene. Mass spectrometry analysis using 13C-labeled CH4 revealed that carbon contained in CH4 was incorporated into the pyrolysis products. The results suggested that CH4 was activated with the aid of C2H6. We assumed that CH4 was attacked by radical species generated from pyrolysis of C2H6 and was converted into methyl radicals. The CH4-derived methyl radicals were incorporated into pyrolysis products via radical reactions. This study clarified that CH4 can be activated by radicals generated from co-existing molecules without the help of catalysts or extremely high temperature.


 Please wait while we load your content...
Please wait while we load your content...