Synthesis of tricyclic carbohydrate–benzene hybrids as selective inhibitors of galectin-1 and galectin-8 N-terminal domains†
Abstract
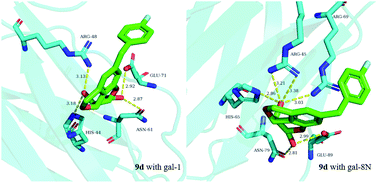
As the galactoside binding family of galectin proteins is involved in many physiological and pathological processes, the inhibitors of these proteins are considered to be of significant interest in the treatment of diseases such as cancer and fibrosis. Herein, fused tricyclic carbohydrate–benzene hybrid core structures are reported to be the selective inhibitors of galectin-1 and the N-terminal domain of galectin-8 by a competitive fluorescence polarization assay. The key intermediates mono- or diiodo tricyclic carbohydrate–benzene hybrids were synthesized from protected 2-bromo-3-O-propargyl-D-galactose via a domino reaction and subsequently utilized for further derivatization by Stille couplings to achieve derivatives carrying substituents at C10 and/or C11. Several compounds showed affinity for the galectin-1 and galectin-8 N-terminal (8N) domains; however, weak or even no binding was observed for galectin-3. Monosubstituted derivatives at C10 or C11 exhibited better affinity for galectin-8N than di-substituted derivatives at C10 or C11. Especially, a benzyl substituent or p-fluorobenzyl substituent at C11 displayed affinity and selectivity for galectin-1 and galectin-8N over galectin-3. This suggests that tricyclic carbohydrate–benzene hybrids are promising scaffolds for the development of selective galectin-1 and galectin-8N inhibitors.


 Please wait while we load your content...
Please wait while we load your content...