Structural properties of the chelating agent 2,6-bis(1-(3-hydroxypropyl)-1,2,3-triazol-4-yl)pyridine: a combined XRD and DFT structural study†
Abstract
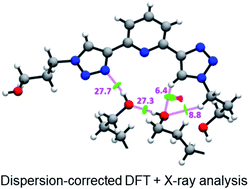
The conformational isomerism of the chelating agent 2,6-bis(1-(3-hydroxypropyl)-1,2,3-triazol-4-yl)pyridine (PTD), exploited in fuel reprocessing in spent nuclear waste, has been studied by single crystal X-ray diffraction analysis in combination with an extensive DFT conformational investigation. In the solid-state, the elucidated crystal structure (i.e., not yet published) shows that by thermal treatment (DSC) no other phases are observed upon crystallization from the melt, indicating that the conformation observed by X-ray data is rather stable. Mapping of intermolecular and intramolecular noncovalent interactions has been used to elucidate the unusual arrangement of the asymmetric unit. Considerations relating to the stability of different conformational isomers in aqueous and non-aqueous solutions are also presented. The accurate structural description reported here might open various research topics such as the potential of PTD to act as an outer sphere ligand in the formation of second sphere coordination complexes and their interconversion by mechanochemical means.


 Please wait while we load your content...
Please wait while we load your content...