A Ln(iii)-3-hydroxypyridine pH responsive probe optimized by DFT†
Abstract
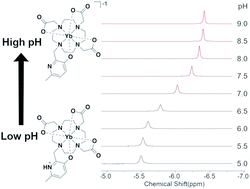
Differences in tissue pH can be diagnostic of cancer and other conditions that shift cell metabolism. Paramagnetic probes are promising tools for pH mapping in vivo using magnetic resonance spectroscopy (MRS) as they provide uniquely shifted MR signals that change with pH. Here, we demonstrate a 3-hydroxy-6-methylpyridyl coordinating group as a new pH-responsive reporter group for Ln(III) MRS probes. The pH response of the complex was observed by UV-Vis, fluorescence, and NMR spectroscopies, and modeled using DFT. These results provide insight into the observed pH-dependent NMR spectrum of the complex. The protonation state of the hydroxypyridine changes the coordinating ability of the ligand, affecting the dipolar field of the lanthanide and the chemical shift of nearby reporter nuclei. The favorable pH response and coordination properties of the 3-hydroxypyridyl group indicate its potential for further development as a dual responsive-reporter group. Incorporation into optimized scaffolds for MRS detection may enable sensitive pH-mapping in vivo.
- This article is part of the themed collection: Shining a Light on the f-Block


 Please wait while we load your content...
Please wait while we load your content...