Two-dimensional square-grid iron(ii) coordination polymers showing anion-dependent spin crossover behavior†
Abstract
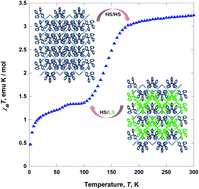
Two Fe(II)-based coordination polymers [Fe(tpmd)2(NCS)2]·5.5H2O (1) and [Fe(tpmd)2(NCSe)2]·7H2O (2) with the framework of square-grid type have been assembled from FeSO4·7H2O, N,N,N′,N′-tetrakis(pyridin-4-yl)methanediamine (tpmd), and KNCS/KNCSe in methanol and characterized. By utilizing two pyridine groups of a tpmd ligand, 1 and 2 are formed in two-dimensional layered structures through coordination of octahedral iron(II) ions with the tpmd to NCS−/NCSe− ligands in which they have a supramolecular isomorphous conformation. 1 shows a paramagnetic behavior between 2 and 300 K, while 2 exhibits two-step spin crossover (ca. 145 and 50 K) in the temperature range due to the coordination of NCSe− ligands. At 300 K 2 is fully high-spin state. However, at 100 K 2 becomes ca. 50% high spin and 50% low spin iron(II) ions, which is verified by magnetic moments. In the structural analysis of 2 at 100 K, two different layers are observed with different bond distances around iron(II) ions in which the layers are stacked alternately.


 Please wait while we load your content...
Please wait while we load your content...