Photocatalytic intermolecular anti-Markovnikov hydroamination of unactivated alkenes with N-hydroxyphthalimide†
Abstract
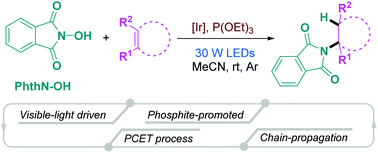
A visible-light-induced/phosphite-promoted anti-Markovnikov hydroamination of alkenes with N-hydroxyphthalimide was successfully realized, which was initiated by a proton-coupled electron transfer to enable direct cleavage of its N–O bond. Both experimental and computational studies were performed to probe the reaction mechanism. A wide range of alkenes, including vinyl ethers, silanes, and sulfides as well as electronically unbiased terminal and internal alkenes, were well tolerated in this hydroamination protocol.


 Please wait while we load your content...
Please wait while we load your content...