Scandium-catalyzed highly selective N2-alkylation of benzotriazoles with cyclohexanones†
Abstract
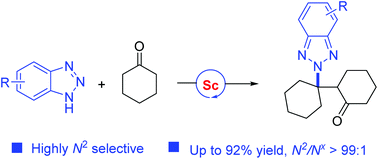
A scandium-catalyzed highly N2-selective alkylation of benzotriazoles with cyclohexanones has been developed, providing straightforward access to a series of N2-alkylated benzotriazoles featuring newly formed tetrasubstituted carbon centers in high yields with excellent regioselectivities. The key to the success of this reaction is using Sc(OTf)3 as the catalyst. Typically, this protocol is also well compatible with 4-aryl-1,2,3-triazoles.


 Please wait while we load your content...
Please wait while we load your content...