Cascade cyclization versus chemoselective reduction: a solvent-controlled product divergence†
Abstract
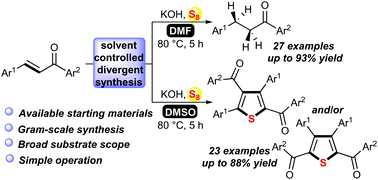
A simple and efficient strategy for the assembly of polysubstituted thiophenes and the chemoselective reduction of α,β-unsaturated ketones via the reaction of enones with elemental sulfur and by a simple switching of the reaction solvent is described. Mechanistic studies revealed that both the reactions proceeded via radical pathways, and two hydrogen atoms came from H2O in the reduction course.


 Please wait while we load your content...
Please wait while we load your content...