Synthesis of polysubstituted quinolines through promoter-regulated selective annulation and C–C bond cleavage from 2-styrylanilines and β-keto esters†
Abstract
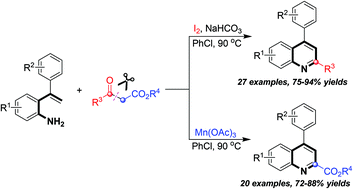
A powerful construction of 2-alkylquinolines and quinoline-2-carboxylates has been successfully accomplished employing I2 and Mn(OAc)3, respectively, to promote the annulation of 2-styrylanilines and β-keto esters accompanied by selective C–C bond cleavage. Following this promoter-regulated strategy, various 2-alkylquinolines and quinoline-2-carboxylates were chemoselectively synthesized from easily available 2-styrylanilines and β-keto esters. The reaction mechanism is also tentatively investigated.


 Please wait while we load your content...
Please wait while we load your content...