Palladium-catalyzed intramolecular aerobic alkenylhydroxylation of allenamides with alkenyl iodides†
Abstract
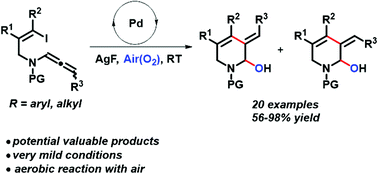
An efficient palladium-catalyzed aerobic alkenylhydroxylation cyclization of allenamide derivatives to synthesize piperidinol derivatives with excellent regioselectivity was developed. Both AgF and THF were proved to be important in the activation of molecular oxygen during this transformation. DFT calculation provided an explanation for the obtained E and Z configurations of the formed double bond, which might be the steric hindrance between two groups on C1 and C7. Mechanistic studies indicated that the reaction might undergo a radical process and a tentative catalytic cycle was proposed.


 Please wait while we load your content...
Please wait while we load your content...