Supramolecular control of unidirectional rotary motion in a sterically overcrowded photoswitchable receptor†
Abstract
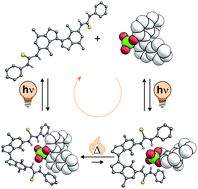
The control of molecular motion by external stimuli has attracted major interest in recent years. In order to achieve unidirectional rotational motion, intrinsically chiral light-driven molecular motors have been shown to be particularly versatile. We recently introduced a system in which unidirectional rotation is achieved in an achiral photoswitchable receptor upon binding of a chiral guest molecule. In order to gain detailed insight into the rotary steps, we present here a modified design in which the steric crowding is increased such that interconversion between the helical isomers of the (Z)-form of the receptor becomes very slow. DFT calculations support the increase in interconversion barrier. Furthermore, two diastereomeric complexes of the chiral guest bound to the (Z)-receptor are distinguished in the 1H NMR spectrum and the ratio between them slowly changes over time as a result of thermal helix inversion. On the other hand, no enrichment in one of the diastereomeric complexes is observed in the photochemical step. Our studies therefore unequivocally confirm that net rotation, induced by the chiral guest, takes place. This study affords an improved understanding of these dynamic supramolecular systems and expands the possibilities to control chirality transfer and motion at the molecular level, spurring developments of supramolecular recognition and information transfer at the nanoscale.
- This article is part of the themed collection: Recent Open Access Articles in Frontiers Journals


 Please wait while we load your content...
Please wait while we load your content...