Redox deracemization of diarylmethyl alkynes†
Abstract
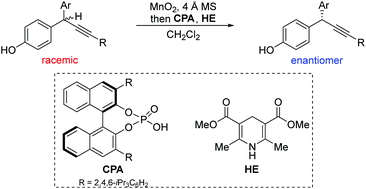
Diarylmethyl alkynes with chirality at the propargylic position are privileged building blocks in many biologically important molecules. However, catalytic asymmetric access to such moieties has remained underdeveloped. Herein, we described a one-pot redox deracemization of diarylmethyl alkynes. By using MnO2 as a mild, inexpensive, and environmentally benign oxidant, combined with chiral phosphoric acid catalyzed asymmetric reduction, deracemization of diarylmethyl alkynes proceeds efficiently with high levels of chemo- and enantioselectivities. The generality of the strategy is further illustrated by redox deracemization of diarylmethyl alkenes.


 Please wait while we load your content...
Please wait while we load your content...