Diastereodivergent aminocatalyzed spirocyclization strategies using 4-alkylideneisoxazol-5-ones and methyl vinyl ketones†
Abstract
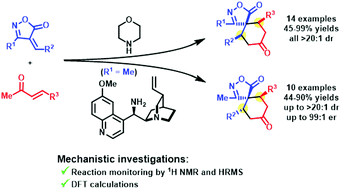
Diasterodivergent aminocatalyzed approaches have been developed for the synthesis of racemic 6,10-cis-spiroisoxazol-5-ones and highly enantioenriched 6,10-trans-spiroisoxazol-5-ones, both protocols starting from 4-alkylideneisoxazol-5-ones and methyl vinyl ketones. Reaction monitoring by 1H NMR and HRMS, and DFT calculations have been performed in order to support a mechanistic rationale explaining such outcomes, depending on the reaction conditions employed.


 Please wait while we load your content...
Please wait while we load your content...