Total and formal syntheses of fostriecin†
Abstract
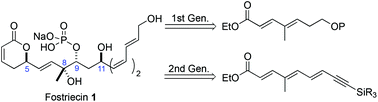
Two formal syntheses and one total synthesis of fostriecin (1) have been achieved, as well as, the synthesis of its related congener dihydro-dephospho-fostriecin. All the routes use the Sharpless dihydroxylation to set the absolute stereochemistry at C-8/9 positions and a Leighton allylation to set the C-5 position of the natural product. In the formal syntheses a Noyori transfer hydrogenation of an ynone was used to set the C-11 position while the total synthesis employed a combination of asymmetric dihydroxylation and Pd-π-allyl reduction to set the C-11 position. Finally in the total synthesis, a trans-hydroboration of the C-12/13 alkyne was used in combination with a Suzuki cross coupling to establish the Z,Z,E-triene of fostriecin (1).
- This article is part of the themed collection: 2020 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...