Nucleophile-assisted cyclization of β-propargylamino acrylic compounds catalyzed by gold(i): a rapid construction of multisubstituted tetrahydropyridines and their fused derivatives†
Abstract
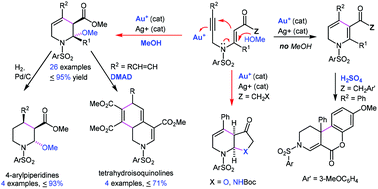
Au(I)-catalyzed cyclization of β-propargylamino acrylic derivatives 1, carried out in the presence of methanol, afforded tetrahydropyridines 3 in high isolated yields. An intramolecular version, using substrates with pending OH, NHBoc and 3-MeOC6H4 groups as internal O-, N-, and C-nucleophiles, yielded the ortho-fused cyclic derivatives, namely furo[2,3-b]pyridine derivative 14, pyrrolo[2,3-b]pyridine 18, and an unusual chromeno[3,4-c]pyridine 22 with a new quaternary carbon center. Synthetic utilization of the tetrahydropyridines 3 has been demonstrated by their conversion into substances with privileged pharmacophore scaffold. Thus, four 4-aryl piperidine derivatives 23a,b,f,g were obtained on catalytic hydrogenation of the representative 4-aryl tetrahydropyridines 3a,b,f,g; furthermore, the Diels–Alder cycloaddition of dimethyl acetylene dicarboxylate (DMAD) to dienes 3r–3u, possessing a masked dendralene framework, afforded tetrahydroisoquinolines 25r–25u that can be aromatized to produce polysubstituted isoquinolines, as demonstrated by the conversion of 25r into 27r. These domino transformations thus offer numerous variations of this methodology and reveal its potential for synthetic applications.


 Please wait while we load your content...
Please wait while we load your content...