Copper-catalyzed tandem phosphorylative allenylation/cyclization of 1-(o-aminophenyl)prop-2-ynols with the P(O)–H species: access to C2-phosphorylmethylindoles†
Abstract
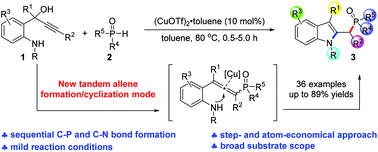
A novel cascade reaction of 1-(o-aminophenyl)prop-2-ynols with the P(O)–H species has been developed, which leads to C2-phosphorylmethylindoles with sequential C–P and C–N bond formation in a single operation. Mechanistic studies suggest that the reaction proceeds via a carbocation initiated tandem phosphorylation/5-exo-dig cyclization, which firstly demonstrates the tandem allene formation/cyclization as a new reaction mode of 1-(o-aminophenyl)prop-2-ynols.


 Please wait while we load your content...
Please wait while we load your content...