Topomeric aza/thia cryptands: synthesis and theoretical aspects of in/out isomerism using n-alkyl bridging†
Abstract
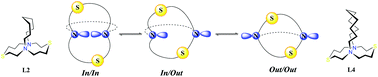
A series of heterobicyclic aza/thia-lactams and cryptands incorporating changes in n-alkyl bridging length have been synthesized, characterized, chelated to heavy metals and computationally assessed. Spectroscopic analysis of aza/thia-lactams L1 and L3 revealed multiple conformational isomers, due to rotameric perturbations associated with the amide bond. Correlation spectroscopy on amine cryptands L2 and L4 supported the presence of intrinsic topological chirality, resulting from a preferred orientation of the amine lone pairs in solution. The unique magnetic environments observed in 1H NMR were attributed to the electron density inside the crypt, which adopt a nephroidal-epicycloid structure with two nitrogen atoms representing the cusps. The major conformational and constitutional isomers for L2 and L4 present in solution were determined to be the in/in pair of enantiomers (R,R and S,S) and confirmed through computational analysis. Application of ligands L1–L4 as heavy metal chelates was addressed using Pb(II), Hg(II), Cd(II) and Ag(I) through NMR spectroscopy and mass spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...