The key role of protodeauration in the gold-catalyzed reaction of 1,3-diynes with pyrrole and indole to form complex heterocycles†
Abstract
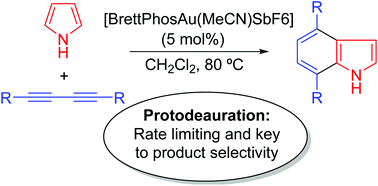
Indole is a very common structural motif in alkaloids, and a number of them feature remarkable bioactive properties. Due to its relevant role in medicinal chemistry and the pharma-industry, many methods for indole synthesis have been developed, but only a few of them exploit the [4 + 2] approach. Recently a successful attempt at indole and carbazole formation via a formal gold mediated [4 + 2] addition of a diyne to pyrrole and indole rings has been reported by Ohno. A number of intriguing features observed in this reaction are however left to be resolved. We study here the mechanism of the Au-catalyzed reaction of 1,3-diynes with pyrrole and indole rings leading to substituted indoles and carbazoles, respectively. The reaction pathways are found to be significantly more complex than we had anticipated, for instance, in the case of the formation of carbazoles, one competitive route involves the formation of an unexpected spirane intermediate that evolves via a 1,2-alkenyl migration. Beyond this complexity, the current study also serves to highlight the importance of fundamental steps that are often disregarded as kinetically irrelevant, such as the protodeauration of reaction intermediates.


 Please wait while we load your content...
Please wait while we load your content...