Total synthesis study of rauvomines A and B: construction of the pentacyclic core structure†
Abstract
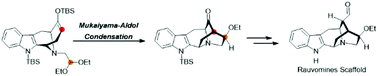
A synthetic route has been developed for the core scaffold of rauvomines, which possess an abnormal sarpagine-type backbone without the C20-methylene substitution. The E-ring annulation was achieved by a TiCl4-catalyzed Mukaiyama-Aldol reaction with a moderate yield of 70%.


 Please wait while we load your content...
Please wait while we load your content...