Differential formation of nitrogen-centered radicals leading to unprecedented, regioselective bromination of N,N′-(1,2-phenylene)bisamides and 2-amidophenols†
Abstract
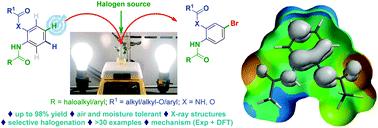
A highly efficient, site-selective, visible light-accelerated, remote C–H halogenation of unsymmetrical aromatic bisamides/amidoesters has been developed. Unprecedented selectivity was realized in this C–H functionalization reaction. The N,N′-(1,2-phenylene)bisamides/amidoesters, derived from various halogenated acids and aliphatic/aromatic acids, were selectively mono-brominated para to the more electron-deficient group. This unique, site-selective bromination of aromatic amine derivatives proceeded under mild and metal-free conditions, without the use of any oxidant or base. The present procedure benefits from short reaction times, avoids excessive bromination, and is air and moisture tolerant. Furthermore, the synthetic utility of the products has been demonstrated by preparing useful substrates and intermediates, including 2-perfluoroalkyl benzimidazole, para-bromoaniline derivatives and substituted biphenyls. A mechanistic investigation using experimental and theoretical means was undertaken to attempt to explain the observed phenomena. Based on DFT calculations, the mechanism involves the differential formation of nitrogen-centered (amidyl) radicals which leads to the observed regioselectivity. Additionally, when the radical stabilizing abilities of both amides are similar, the regioselectivity breaks down, leading to a mixture of products (2x–2z).


 Please wait while we load your content...
Please wait while we load your content...