Toward C2-nitrogenated chromones by copper-catalyzed β-C(sp2)–H N-heteroarylation of enaminones†
Abstract
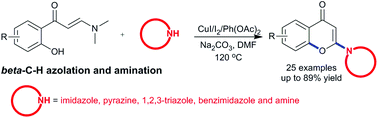
The unconventionally selective β-C(sp2)–H activation of enaminones in the presence of various nitrogen nucleophiles, including azoles and cyclic amines, leading to the synthesis of 2-nitrogenated chromones has been achieved by employing a catalytic system consisting of CuI, I2, PhI(OAc)2 and Na2CO3. The work discloses a new application of functonal enaminones in the synthesis of valuable heterocyclic scaffolds by switching the reaction pathway via unprecedented regioselectivity.


 Please wait while we load your content...
Please wait while we load your content...