Catalytic asymmetric 1,4-type Friedel–Crafts (hetero)arylations of 1-azadienes: the highly enantioselective syntheses of chiral hetero-triarylmethanes†
Abstract
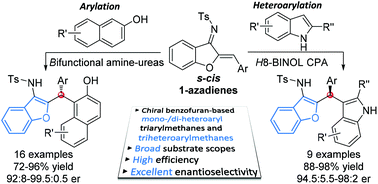
Direct enantioselective Michael-type Friedel–Crafts arylations and heteroarylations of s-cis 1-azadienes were achieved by applying chiral bifunctional tertiary amine-urea catalysts when using 2-naphthols as the nucleophiles, and phosphoric acid catalysts when using indoles as the nucleophiles. These two catalytic protocols efficiently enabled access to a diverse variety of important benzofuran-containing hetero-triarylmethanes in up to 98% yield and with 99.5 : 0.5 er.


 Please wait while we load your content...
Please wait while we load your content...