Reductive coupling of imines with redox-active esters by visible light photoredox organocatalysis†
Abstract
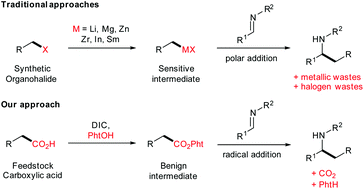
The addition of organometallic compounds to imines is a direct way for accessing α-branched secondary amines which are found in numerous bioactive molecules. Although convenient, such reactions typically involve the formation and isolation of the nucleophile in the case of Grignard reactions, or the in situ formation of the nucleophile for Barbier reactions, leading to stoichiometric amounts of metallic wastes. Herein, we report the direct alkylation of imines with redox-active esters by visible light photoorganocatalysis. With Rose bengal as inexpensive photocatalyst, and green light as sustainable energy source, the synthesis of a wide range of amines and θ-amino esters was achieved from inexpensive feedstock chemicals, in a highly modular fashion.


 Please wait while we load your content...
Please wait while we load your content...