A dienamine-mediated deconjugative addition/cyclization cascade of γ,γ-disubstituted enals with carboxylic acid-activated enones: a rapid access to highly functionalized γ-lactones†
Abstract
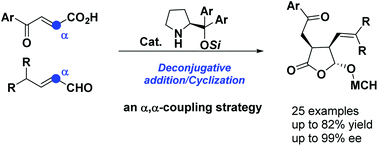
An aminocatalytic deconjugative addition/cyclization cascade of γ,γ-disubstituted enals with carboxylic acid-activated enones was realized. The in situ generated dienamine species was regioselectively trapped at the α position by carboxylic acid-activated enones, leading to highly functionalized γ-lactones with excellent enantioselectivities. Further synthetic elaboration of the products allows diverse synthesis of a set of complex chiral targets.


 Please wait while we load your content...
Please wait while we load your content...