A combined experimental and computational study of NHC-promoted desulfonylation of tosylated aldimines†
Abstract
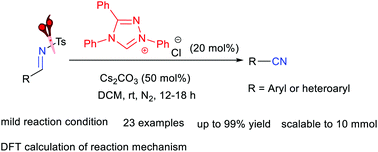
NHC-catalyzed desulfonylation of tosylated aldimines provides facile access to aryl nitriles in generally good to excellent yields with good functional group compatibilities under mild reaction conditions. DFT calculations demonstrate that the reaction undergoes multiple stepwise processes including nucleophilic addition of NHC to tosylated imine, formation of an aza-Breslow intermediate, deprotonation, and dissociation of the tosyl group.


 Please wait while we load your content...
Please wait while we load your content...