Synthesis of α-cyano sulfone via thermal rearrangement of 1,4-disubstituted triazole mediated by carbene and radical species†
Abstract
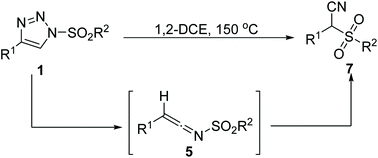
A novel metal-free rearrangement of 4-substituted 1-sulfonyl-1,2,3-triazoles producing α-cyano sulfones in satisfactory yields is reported. A series of control experiments have been conducted. Carbene and radical species were proposed as the key intermediates and ketenimine was captured by an intramolecular reaction. This process is fundamentally different from that reported in the literature which produces the same product. Several fused multi-cycle compounds could be constructed efficiently.


 Please wait while we load your content...
Please wait while we load your content...