Bistachybotrysins L–V, bioactive phenylspirodrimane dimers from the fungus Stachybotrys chartarum†
Abstract
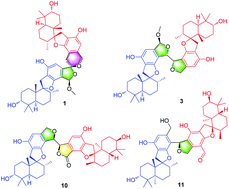
Bistachybotrysins L–V (1–11), eleven novel dimeric phenylspirodrimanes, were isolated from the fungus Stachybotrys chartarum CGMCC 3.5365. Among them, 1 and 2, a pair of stereoisomeric dimers represent an unusual aromatic [5,6]-spiroketal skeleton with a central 2-methoxy-1,6-dioxaspiro[4.5]decane core fused with two phenyl units. Their structures, including absolute configurations, were elucidated using extensive spectroscopic data, single-crystal X-ray diffraction (Cu Kα), and calculated electronic circular dichroism (ECD) analyses. Biological assay revealed that 2 showed potent cytotoxicity against five human tumor cell lines with IC50 values in the range of 1.8–3.5 μM; 2, 3, and 9 displayed neuroprotective effects toward the glutamate-induced toxicity in SK-N-SH cells by increasing cell viability 17.4%–17.6% at 10.0 μM; and 8 exhibited anti-inflammatory activity by suppressing LPS-induced NO production in BV2 cells with an inhibition rate of 54.2% at 10.0 μM. Furthermore, the possible biogenetic pathways of these compounds are proposed.


 Please wait while we load your content...
Please wait while we load your content...