Hydrogen peroxide disproportionation with manganese macrocyclic complexes of cyclen and pyclen†
Abstract
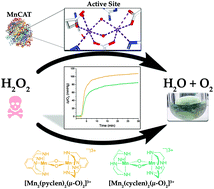
The catalase family of enzymes, which include a variety with a binuclear manganese active site, mitigate the risk from reactive oxygen species by facilitating the disproportionation of hydrogen peroxide into molecular oxygen and water. In this work, hydrogen peroxide disproportionation using complexes formed between manganese and cyclen or pyclen were investigated due to the spectroscopic similarities with the native MnCAT enzyme. Potentiometric titrations were used to construct speciation diagrams that identify the manganese complex compositions at different pH values. Each complex behaves as a functional mimic of catalase enzymes. UV-visible spectroscopic investigations of the H2O2 decomposition reaction yielded information about the structure of the initial catalyst and intermediates that include monomeric and dimeric species. The results indicate that rigidity imparted by the pyridine ring of pyclen is a key factor in increased TON and TOF values measured compared to cyclen.


 Please wait while we load your content...
Please wait while we load your content...
