Understanding structure–property relationships of main chain cyclopropane in linear polyesters†
Abstract
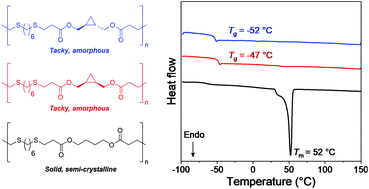
Rigid ring structures have gained increasing interest in the polymer materials community as an effective means to manipulate bulk properties. Despite this, little work has focussed on the smallest ring: cyclopropane. Herein, we report a polymerisation that enables incorporation of stereopure cis and trans 1,2-cyclopropanedimethanol through the thiol-Michael “click” reaction between dithiol and diacrylate monomers. Polyesters containing a cyclopropane backbone were found to be amorphous, whilst comparable polymers with a 1,4-butanediol backbone were semi-crystalline. By copolymerising cyclopropane monomers at varying ratios with 1,4-butanediol monomers, the crystalinity of the resulting polymer could be effectively tuned. Successfully adjusting the resulting crystallinity resulted in control over the ultimate tensile strength (UTS) and the Young's modulus (E) of the material. Generally, it was found that increasing cyclopropane content led to a decreased UTS and E as a result of lower polymer crystalinity. Effects of cyclopropane stereochemistry on thermomechanical properties were found to be minimal at low cyclopropane backbone ratios.


 Please wait while we load your content...
Please wait while we load your content...