Synthesis of an optically active polymer containing a planar phthalimide backbone by asymmetric polymerization†
Abstract
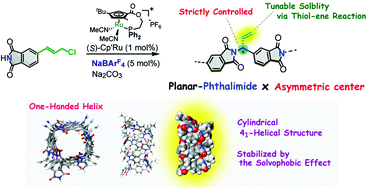
Asymmetric polymerization is a promising method for constructing sophisticated backbones that contain asymmetric carbons on the main chain. Herein, we report the precise design and synthesis of a novel polymer backbone that induces a helical structure through asymmetric polymerization reactions of a phthalimide-based monomer catalyzed by a planar-chiral cyclopentadienyl–ruthenium complex. When sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate was used as an additive, the polymerization proceeded with high regio- and enantioselectivity, producing an optically active polymer composed of a planar phthalimide backbone with enantiomerically enriched asymmetric carbons on the main chain. The terminal olefin in each monomer unit of the polymer could take on various substituents through thiol–ene reactions. CD and UV spectroscopic methods and conformational studies showed that the planar phthalimide backbones are tightly folded according to the local conformation determined by the two torsion angles around the asymmetric carbons of the main chain, resulting in the construction of a cylindrical 41-helical structure in solution, which was stable in polar solvents due to the solvophobic effect on the phthalimide backbones.


 Please wait while we load your content...
Please wait while we load your content...