Semifluorinated, kinked polyarylenes via direct arylation polycondensation†
Abstract
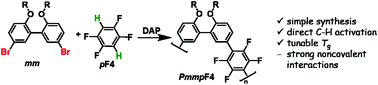
Semifluorinated, amorphous polyarylenes PmmpF4 with kinked backbone structure were prepared from a meta-substituted, biphenol-based monomer with varying alkoxy substituents R and 1,2,4,5-tetrafluorobenzene (pF4) via direct arylation polymerization (DAP). The chemistry employed is simple, scalable and does not rely on tedious purification techniques. Polycondensation occurs cleanly without major side reactions. Despite the clean polycondensation reaction, very high molar mass materials are difficult to obtain, which is ascribed to an unusual solubility behavior compared to non-fluorinated analogs, and similar, yet more linear tetrafluorobenzene copolymers based on fluorene or carbazole. In order to investigate this phenomenon further, the side chain-dependent properties PmmpF4 are investigated using linear, branched and cyclic side-chains. While the glass transition temperature of PmmpF4 is a strong function of R and can be varied between 35 °C and 197 °C for constant backbone structure and molecular weight, solubility cannot be improved by using longer linear or branched side chains. Density functional theory calculations suggest significant polarization-type non-covalent interactions between tetrafluorobenzene and the biphenol-based monomer as origin for the observed limited solubility, which guide the design of both kinked and straight conjugated polymers with high molar mass and solubility.
- This article is part of the themed collection: Polymer Chemistry Pioneering Investigators 2021


 Please wait while we load your content...
Please wait while we load your content...