A polyesteramide library from dicarboxylic acids and 2,2′-bis(2-oxazoline): synthesis, characterization, nanoparticle formulation and molecular dynamics simulations†
Abstract
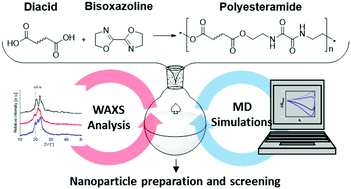
The step growth polyaddition of a variety of dicarboxylic acids and 2,2′-bis(2-oxazoline) enabled access to a library of polyesteramides (PEA) with different linker lengths or bulkiness. PEA with alkylene spacers represented semicrystalline materials due to hydrogen bond formation between the oxamide moieties, as is evident from dynamic scanning calorimetry and wide-angle X-ray scattering (145 °C < Tm < 225 °C). In contrast, PEAs comprising substituted spacers resulted in amorphous materials suitable for the preparation of nanoparticle dispersions by means of direct nanoprecipitation from hexafluoroisopropanol into water. Strong intermolecular interactions that were evident from Hildebrand solubility parameters δ resulted in the formation of aggregates for the semi-crystalline materials. Although Hildebrand parameters alone provided less accurate solubility predictions of the PEAs in acetone and THF, the Flory–Huggins parameters χPS revealed pronounced changes of intermolecular interactions with variation of the polymer structure, clearly demonstrating a tunable hydrophobicity of the synthesized PEAs.


 Please wait while we load your content...
Please wait while we load your content...