Stoichiometric imbalance-promoted step-growth polymerization based on self-accelerating 1,3-dipolar cycloaddition click reactions†
Abstract
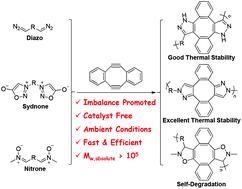
A library of self-accelerating click reactions was developed based on the 1,3-dipolar cycloaddition of sym-dibenzo-1,5-cyclooctadiene-3,7-diyne (DIBOD) and varied 1,3-dipoles, such as diazo, sydnone, and nitrone groups. A common feature of these reactions was that the reaction of a 1,3-dipole and the first alkyne moiety of DIBOD activated in situ the second alkyne moiety, which consequently reacted with a 1,3-dipole at a much faster rate than did the original DIBOD alkyne group. Because these were polymerization reactions, a novel kind of stoichiometric imbalance-promoted step-growth polymerization method was developed specifically to prepare high molecular weight (>105 g mol−1) polymers containing five-membered heterocycles inside polymer backbones. The self-accelerating property of the DIBOD-based 1,3-dipolar cycloadditions enabled the use of step-growth polymerization to prepare high molecular weight polymers under stoichiometric imbalance conditions using an excess of DIBOD over bis-dipole monomers. The click characteristics of the DIBOD-based 1,3-dipolar cycloadditions assisted the step-growth polymerization so that polymers could be prepared under ambient and catalyst-free conditions. In addition, the varied five-membered heterocycle structures inside the backbones endowed the resultant polymers with distinctly unique properties and functions. The polymers with isoxazoline groups inside the backbones demonstrated self-degradation behavior, where higher molecular weights resulted in greater degradation. The polymers with pyrazole groups inside the backbones had excellent thermal properties: the decomposition temperature at 5% weight loss could reach 576 °C, the glass transition temperature could not be measured up to 400 °C, and the char yield at 800 °C was as high as 71%.


 Please wait while we load your content...
Please wait while we load your content...