Synthesis and oligomerization of cysteinyl nucleosides†
Abstract
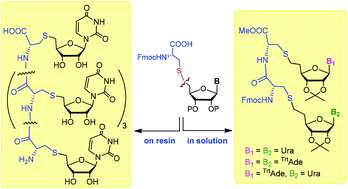
Nucleoside and nucleic acid analogues are known to possess a considerable therapeutic potential. In this work, by coupling cysteine to nucleosides, we successfully synthesized compounds that may not only have interesting biological properties in their monomeric form, but can be used beyond that, for oligomerization, in order to produce new types of synthetic nucleic acids. We elaborated different strategies for the synthesis of cysteinyl nucleosides as monomers of cysteinyl nucleic acids using nucleophilic substitution or thiol–ene coupling as a synthetic tool, and utilised on two complementary nucleosides, uridine and adenosine. Dipeptidyl dinucleosides and pentameric cysteinyl uridine were prepared from the monomeric building blocks, which are the first members of a new class of peptide nucleic acids containing the entire ribofuranosyl nucleoside units bound to the peptide backbone.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...