Visible-light-enabled aerobic oxidative Csp3–H functionalization of glycine derivatives using an organic photocatalyst: access to substituted quinoline-2-carboxylates†
Abstract
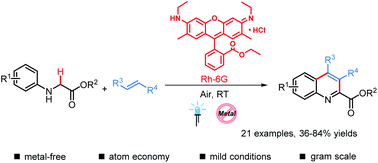
A practical visible-light-induced aerobic oxidative dehydrogenative coupling reaction of glycine derivatives with olefins has been developed to efficiently synthesize quinoline-2-carboxylates. This metal-free process proceeds smoothly under mild conditions and exhibits good tolerance of functional groups. Given the low cost of the catalyst and feedstock materials, the mild reaction conditions and the absence of hazardous byproducts, this protocol should find broad applications in the synthesis of quinoline-2-carboxylate derivatives.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...