Thiophene-based water-soluble fullerene derivatives as highly potent antiherpetic pharmaceuticals†
Abstract
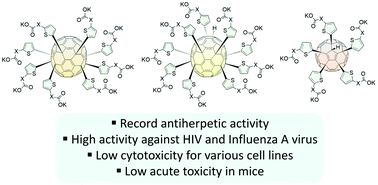
Here we report the Friedel–Crafts arylation of chlorofullerenes C60Cl6 and C70Cl8 with thiophene-based methyl esters. While C60Cl6 formed expected Cs-C60R5Cl products, C70Cl8 demonstrated a tendency for both substitution of chlorine atoms and addition of an extra thiophene unit, thus forming Cs-C70R8 and C1-C70R9H compounds. The synthesized water-soluble C60 and C70 fullerene derivatives with thiophene-based addends demonstrated high activity against a broad range of viruses, including human immunodeficiency virus, influenza virus, cytomegalovirus, and herpes simplex virus. The record activity of C70 fullerene derivatives against herpes simplex virus together with low toxicity in mice makes them promising candidates for the development of novel non-nucleoside antiherpetic drugs.


 Please wait while we load your content...
Please wait while we load your content...