Total chemical synthesis of the phosphorylated p62 UBA domain reveals that Ser407Pi but not Ser403Pi enhances ubiquitin binding†
Abstract
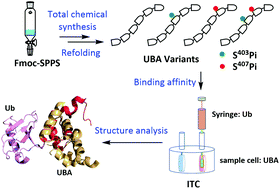
As an autophagic adaptor, p62 specifically targets ubiquitinated proteins to an autophagosome for lysosomal degradation through a critical ubiquitin-associated (UBA) domain. Recent research studies reported that the Ser403 and Ser407 sites on the UBA domain were modified by phosphorylation, increasing the binding affinity between p62 and ubiquitin (Ub). However, the exact role of each phosphorylation site in the regulation of the UBA domain and Ub binding remains unclear. In this text, we applied total chemical synthesis to prepare four types of phosphorylated UBAs, among which the bisphosphorylated UBA was successfully synthesized via the pseudo-dipeptide unit and auxiliary-mediated hydrazide-based native chemical ligation (NCL). Isothermal titration calorimetry (ITC) assays showed that the phosphorylation at S407 enhanced the binding affinity between UBA and Ub, while that at S403 did not. It was suggested that phosphorylation at S407 might be important for promoting the interplay between the UBA domain and Ub, whereas phosphorylation at S403 was not directly involved in this interaction.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...