Concise asymmetric synthesis of new enantiomeric C-alkyl pyrrolidines acting as pharmacological chaperones against Gaucher disease†
Abstract
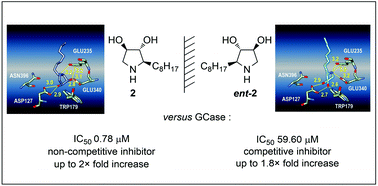
A concise and asymmetric synthesis of the enantiomeric pyrrolidines 2 and ent-2 are herein reported. Both enantiomers were assessed as β-GCase inhibitors. While compound ent-2 acted as a poor competitive inhibitor, its enantiomer 2 proved to be a potent non-competitive inhibitor. Docking studies were carried out to substantiate their respective protein binding mode. Both pyrrolidines were also able to enhance lysosomal β-GCase residual activity in N370S homozygous Gaucher fibroblasts. Notably, the non-competitive inhibitor 2 displayed an enzyme activity enhancement comparable to that of reference compounds IFG and NN-DNJ. This work highlights the impact of inhibitors chirality on their protein binding mode and shows that, beyond competitive inhibitors, the study of non-competitive ones can lead to the identification of new relevant parmacological chaperones.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...