Fatty alcohol synthesis from fatty acids at mild temperature by subsequent enzymatic esterification and metal-catalyzed hydrogenation
Abstract
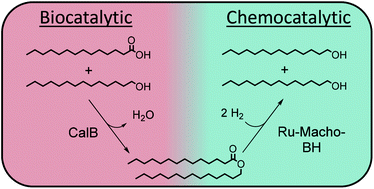
Fatty alcohols are important products in chemical industry to be used in the formulation of surfactants and lubricants. This work describes a two step approach for the production of myristyl alcohol under neat conditions by combining a lipase catalyzed esterification of myristic acid and myristyl alcohol with a ruthenium catalyzed hydrogenation of the intermediate myristyl myristate. The esterification was carried out in a bubble column reactor with the commercial immobilized lipase B from Candida antarctica as a biocatalyst, while the hydrogenation was conducted under pressurized conditions being catalyzed by the homogeneous chemocatalyst Ru-Macho-BH. By investigating the reaction steps separately, comparable reaction rates were found for the esterification of short chain and long chain alcohols. Additionally, the hydrogen pressure could be reduced to 35 bar compared to the current industrial Lurgi process. Characterization of cross interactions by the reactants myristic acid and sodium myristate in the hydrogenation demonstrates that the metal catalyst was completely deactivated, even at a low amount of 0.5 mol% of myristic acid. Complete conversion of myristic acid in the esterification with equal amounts of myristic acid and myristyl alcohol was obtained, overcoming any limitation in the hydrogenation. In comparison to the Lurgi process starting also from fatty acid and fatty alcohols, the chemoenzymatic two step reaction sequence could be realized at lower reaction temperatures of 60 and 100 °C as well as lower hydrogen pressures of 35 bar.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...