Palladium-catalyzed regio- and stereoselective access to allyl ureas/carbamates: facile synthesis of imidazolidinones and oxazepinones†
Abstract
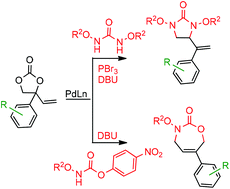
Typically, transition metal catalysis enforces the stereodefined outcome of a reaction. Here we disclose the palladium-catalyzed regio- and stereoselective access to allylic ureas/carbamates and their further exploitation to diverse cyclic structures under operationally simple reaction conditions. This protocol features palladium-catalyzed decarboxylative amidation of highly modular VECs with good to excellent yield, minimal waste production, wide substrate scope, and low catalyst loading. In follow-up chemistry, we demonstrated the debenzylation of vinylic imidazolidinones to N-hydroxycyclic ureas and regioselective derivatization towards the facile synthesis of halohydrins and oxiranes under mild reaction conditions in good to excellent yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...