Metal-free C3–H acylation of quinoxalin-2(1H)-ones with α-oxo-carboxylic acids†
Abstract
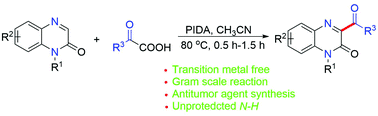
Direct C3–H acylation of quinoxalin-2(1H)-ones with α-oxocarboxylic acids under thermo conditions promoted by PIDA has been achieved in a moderate to good yield in a very fast manner. Mechanistic study revealed that the reaction proceeds via a radical process. In addition, this method could be applied to a gram-scale reaction and antitumor agent synthesis. This work represents a simple, convenient and efficient synthesis of 3-acylated quinoxalin-2(1H)-ones.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...