Use of remote acyl groups for stereoselective 1,2-cis-glycosylation with fluorinated glucosazide thiodonors†
Abstract
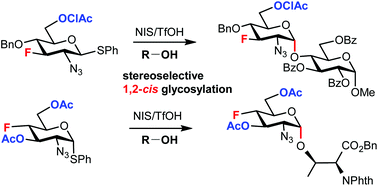
Fluorinated glycans are valuable probes for studying carbohydrate–protein interactions at the atomic level. Glucosamine is a ubiquitous component of glycans, and the stereoselective synthesis of α-linked fluorinated glucosamine is a challenge associated with the chemical synthesis of fluorinated glycans. We found that introducing a 6-O-acyl protecting group onto 3-fluoro and 4-fluoro glucosazide thiodonors endowed them with moderate α-selectivity in the glycosylation of carbohydrate acceptors, which was further improved by adjusting the acceptor reactivity via O-benzoylation. Excellent stereoselectivity was achieved for 3,6-di-O-acyl-4-fluoro analogues. The glycosylation of threonine-derived acceptors enabled the stereoselective synthesis of the protected fluorinated analogue of α-GlcNAc-O-Thr, a moiety abundant in cell-surface O-glycans of the protozoan parasite Trypanosoma cruzi. DFT calculations supported the involvement of transient cationic species which resulted from the stabilization of the oxocarbenium ion through O-6 acyl group participation.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...