On the development of a nucleophilic methylthiolation methodology†
Abstract
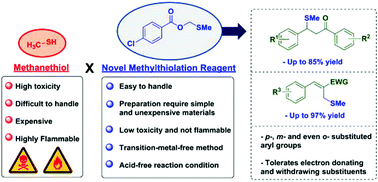
Methylthiolation reactions are usually explored to access organosulfur compounds using methanethiol, an extremely flammable and toxic compound. Herein, methylthiomethyl esters were successfully applied as novel methylthiolation reagents in a low cost, transition-metal-free methodology. These reagents allowed the methylthiolation of a wide scope of chalcones, acyl ester derivatives and Morita–Baylis–Hillman acetates with good group tolerance, affording the methylthiolated products in moderate to excellent yields. The reaction mechanism was investigated through several control experiments, as well as by theoretical calculations employing Density Functional Theory. The results strongly support that a sulfurane and a sulfonium ylide appear as key intermediates and that a Pummerer type rearrangement is also crucial for the formation of this novel reagent. Furthermore, the methylthiolation mechanism is likely to proceed through the nucleophilic attack of the reagent, followed by an entropically favoured step involving the acetate attack to the positively charged species, then releasing the product.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...