Efficient synthesis of a galectin inhibitor clinical candidate (TD139) using a Payne rearrangement/azidation reaction cascade†
Abstract
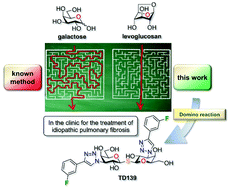
Selective galectin inhibitors are valuable research tools and could also be used as drug candidates. In that context, TD139, a thiodigalactoside galectin-3 inhibitor, is currently being evaluated clinically for the treatment of idiopathic pulmonary fibrosis. Herein, we describe a new strategy for the preparation of TD139. Starting from inexpensive levoglucosan, we used a rarely employed reaction cascade: Payne rearrangement/azidation process leading to 3-azido-galactopyranose. The latter intermediate was efficiently converted into TD139 in a few simple and practical steps.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...