Understanding the mechanism and reactivity of Pd-catalyzed C–P bond metathesis of aryl phosphines: a computational study†
Abstract
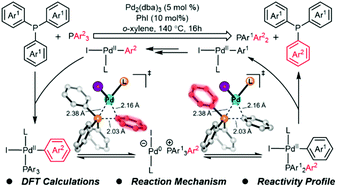
Transition metal-catalyzed single bond metathesis has recently emerged as a useful strategy for functional group transfer. In this work, we explored the mechanism and reactivity profile of Pd/PhI-cocatalyzed C–P bond metathesis between aryl phosphines using density functional theory (DFT) calculations. The overall single bond metathesis involves two Pd(II)-catalyzed C–P reductive eliminations and two Pd(0)-catalyzed C–P oxidative additions, which allows the reversible C–P bond cleavage and formation of the phosphonium cation. Distortion/interaction analysis indicates that the facile C–P bond cleavage and formation of the phosphonium cation are due to the involvement of coordinating aryl phosphine in the process. In addition, the substituent effects on the reaction kinetics and thermodynamics of metathesis were computed, which provides helpful mechanistic information for the design of related single bond metathesis reactions.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...