Substrate-oriented selectivity in the Mg-mediated conjugate addition of bromoform to electron-deficient alkenes†
Abstract
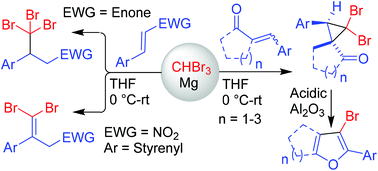
The Mg-mediated conjugate addition of bromoform to a variety of electron-deficient alkenes has been investigated. In the case of nitrodienes and dibenzylideneacetones, tribromomethylated products were isolated, whereas spiro-cyclopropanated products were obtained with cyclic dibenzylideneketones and 3-olefinic oxindoles. The spiro-cyclopropyl ketones derived from cyclic dibenzylideneketones were successfully transformed into fused furans via the Cloke–Wilson rearrangement.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...